Every research project needs an expert helping hand from time to time—whether in the office, at the bench, or in the field.

As a business owner, I manage large research and writing projects for international global health organizations, governmental agencies, and academic and medical institutions. I have extensive experience in writing and editing grant proposals, many of which have been successfully funded. I have assisted with proposals to governmental agencies (e.g., National Institutes of Health [NIH], National Science Foundation [NSF]), Department of Defense [DOD], Centers for Disease Control and Prevention [CDC], and public health foundations (e.g., Bill & Melinda Gates Foundation and PATH). Also, I have aided clients with multi-institutional grant proposals and numerous NIH training grant proposals.
For over 18 years, I have led and conducted research on emerging infectious diseases and diseases related to inadequate access to water, sanitation, and hygiene (WASH).
My emerging infectious disease research was conducted with several academic institutions, international governmental partners, the Centers for Disease Control and Prevention (CDC), and the Defense Threat Reduction Agency (DTRA). I have managed and worked on several WASH-related disease projects with the World Bank, the Wellcome Trust, PATH, and the Bill & Melinda Gates Foundation. Professionals in medicine and academia have relied on my expertise to guide their work to publication in peer-reviewed journals highly regarded in their fields, such as The Lancet Global Health, Vaccine, Nature Communications, Cell, Clinical Epigenetics, Journal of Wildlife Diseases, Vector-Borne and Zoonotic Diseases, the PLOS journals, and others.
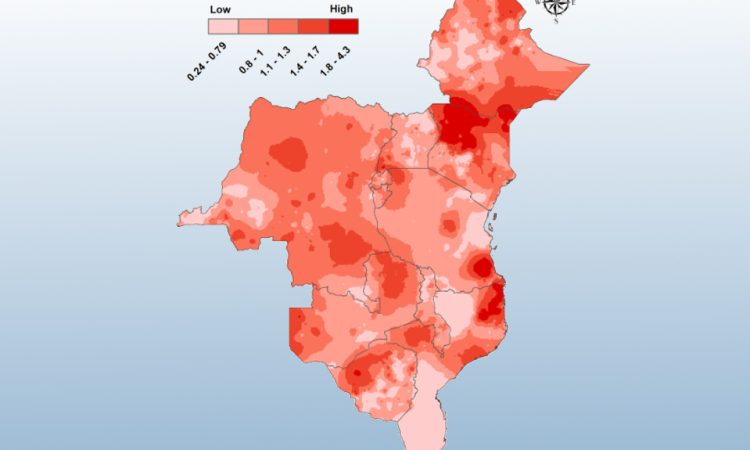
Worldwide, diarrheal diseases—which are largely preventable—are a leading cause of mortality for children under five years old. This is usually a result of poor access to clean water, adequate sanitation, and nutrition. In addition to interventions, targeted vaccination of the most vulnerable children may improve their survival and quality of life.

Hantaviruses are hosted by rodents and insectivores worldwide; some species are transmitted to humans and can cause severe disease. Sin Nombre virus (SNV) and other New World hantaviruses have case fatality rates around 37%–40% and have no vaccine or cure. Understanding the transmission of this family of viruses can help better predict outbreaks and provide insight into other primarily rodent-borne diseases.
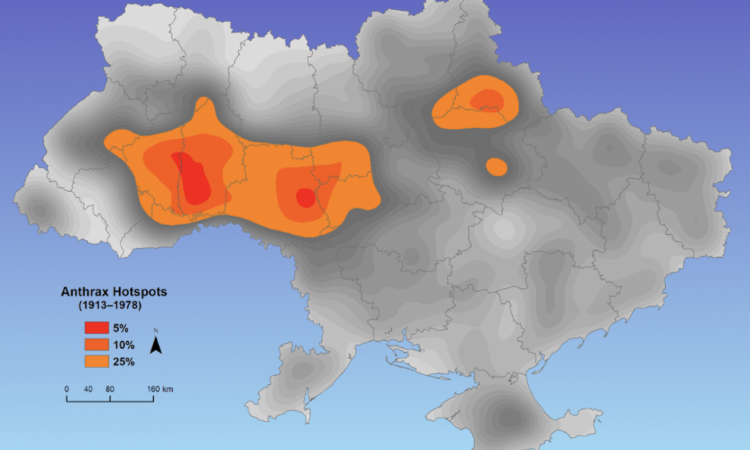
Anthrax, caused by Bacillus anthracis, is an acute disease affecting wildlife, livestock, and humans worldwide, although its impact on these populations is underappreciated. Characterizing the spatial distribution of anthrax and assessing wildlife exposure to this pathogen can help produce more accurate assessments of human risk in the environments in which it persists.

Madariaga virus recently emerged as a human disease in Panamá. Previously unknown to cause disease in humans, in 2010 several children experienced grave neurological symptoms and even death as a result of infection by this mosquito-borne virus. To better understand and ideally eventually prevent this disease, it is crucial to identify its natural host and transmission cycles and how human actions affect its spread.
Water Supply, Sanitation, and Hygiene (WASH) Poverty Diagnostic Initiative | World Bank Group
Our team analyzes economic and geographic heterogeneity in diarrheal mortality risk and burden due to inadequate WASH.
The output of our analyses are used to better target WASH interventions for the subpopulations who need them most within LMICs.
As a part of a larger team, we completed assessments of the total diarrheal disease burden associated with ETEC and Shigella infections for low- to middle-income countries (LMICs). We also provided rotavirus burden heterogeneity and cost-effectiveness analyses to inform vaccine deployment strategies by international governments.
Hantaviruses are hosted by rodents and insectivores worldwide. Some viral species, such as Sin Nombre virus (SNV), can cause severe disease with case fatality rates around 37% to 40% and have no vaccine or cure. Since its identification in a 1993 outbreak in the Southwestern US, there has been extensive research to identify and predict the natural transmission patterns of SNV to prevent this disease in humans.
Designed and implemented large-scale field transmission experiments across multiple institutions (CDC, Emory University, and Montana Tech).
Funded field transmission project through obtaining several competitive grants and fellowships.
Resulted in novel findings about SNV transmission and infection in natural deer mouse populations and in the development of new molecular disease detection and evolutionary analysis tools
Ongoing projects are using these techniques and tools to examine the relationship of rodent community diversity to host stress and viral transmission, as well as to explore SNV evolution in deer mice throughout Montana across a decade of surveillance.
We worked on high-consequence bacterial zoonotic diseases as a part of a US Department of Defense-funded program. This program included engagement with, and training of, international public health scientists and officials from Ukraine, the Republic of Georgia, and certain Central Asian countries. The goal of this research was to better understand the spatial epidemiology of the studied zoonotic diseases and to make related public health recommendations to in-country partners.
While conducting this research, we trained international public health professionals in specialized geographic information system (GIS) techniques to assess spatio-temporal patterns of outbreaks in livestock and wildlife in their home countries. We also led a project exploring new serological techniques to assess anthrax exposure of wildlife at home (Northwestern US) and abroad.
In the past 20 years, several mosquito-borne viruses have become greater threats to human health worldwide. For example, while Zika virus was a known human pathogen prior to 2015, cases were rare, usually associated with mild illness, and geographically limited. Since the 2015 Brazilian outbreak, when Zika virus was first linked to microencephaly in infants, outbreaks and transmission of this flavivirus have been documented in over 80 countries.
Zika’s emergence and other similar stories drive home the importance of understanding and monitoring zoonotic diseases, especially those showing evidence of emerging in human populations. In Panamá, infection by Madariaga virus—previously unknown to cause disease in humans—resulted in several children experiencing grave neurological symptoms and even death. To prevent this alphavirus from establishing itself as a human pathogen, ongoing public health, medical, and entomological research aims to understand its natural transmission cycle. While there are suspected mammalian reservoir hosts and mosquito vectors, they have yet to be unequivocally confirmed, and the hunt for the hosts and vectors of Madariaga virus continues.
Providing expertise related to field transmission experiment design.
Aiding in proposal and grant writing to obtain funding for field transmission experiments geared at finding the reservoir host(s) of this emerging disease.
Delivering field and molecular laboratory assistance in Panamá during mosquito vector trapping and blood meal analysis.
Conducting spatial and other statistical analyses of data collected during these outbreaks.
